Methylenetetrahydrofolate reductase (MTHFR) is one of the most important enzymes in human physiology, having influence on at least as many biochemical processes as it has syllables in its nearly unpronounceable name.
Deficiencies in production or function of this enzyme have been associated with increased risk of myocardial infarction, stroke, venous thrombosis, several types of cancer, congenital defects, inflammatory bowel disease, and several neuropsychiatric conditions. In practice, MTHFR function is an important predictor of predispositions to chronic disease states, and interventions aimed at optimizing MTHFR function can often be preventive or therapeutic
Put most simply, MTHFR converts 5,10-methylenetetrahydrofolate into the activated form, 5-MTHF or 5-methyltetrahydrofolate. Though this reaction plays a part in many biochemical pathways, it is probably best-known in the context of breaking down the amino acid homocysteine. This process produces methionine and eventually S-Adenosylmethionine (SAMe), a crucial DNA methylator.
Other significant roles of a properly functioning MTHFR enzyme include nucleic acid biosynthesis, neurotransmitter synthesis, and production of signaling molecules important for regulating embryonic development, all of which will be discussed in more detail in later sections.
The role of MTHFR in health and disease has been the subject of intense research in recent years, and this work is beginning to influence clinical practice. In many ways, MTHFR function provides important clues to the risk of developing particular diseases, and to the etiology of seemingly unexplained or unexpected symptom patterns.
Fortunately, it is now possible and practical to test for the presence of mutations in the gene coding for this important enzyme.
MTHFR Mutations
The normal wild type (CC) MTHFR gene gives instruction for production of the methylenetetrahydrofolate reductase enzyme. Currently, over 40 point mutations of this gene have been identified. Of these, mutations on the points at C677T and A1298C seem to have the most clinical significance.
In particular, the C677T polymorphism shows a wide regional and ethnic variation. 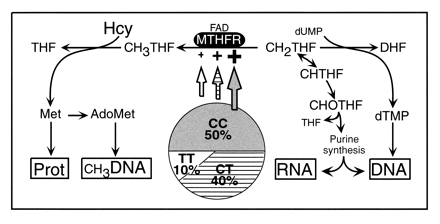 Homozygosity (TT) among Whites is 6-14%. In African populations and in Blacks living outside of Africa such as in Brazil and in the United States, the frequency falls to less than 2% for the TT variant. The prevalence rises in Mediterranean and Hispanic population. For example, among Hispanics in prevalence ranges as high as 21%. Northern China and Japan show an 18 and 12% prevalence respectively (R. Castro, et al. J Med Genet. 2004;41:454-458).
Homozygosity (TT) among Whites is 6-14%. In African populations and in Blacks living outside of Africa such as in Brazil and in the United States, the frequency falls to less than 2% for the TT variant. The prevalence rises in Mediterranean and Hispanic population. For example, among Hispanics in prevalence ranges as high as 21%. Northern China and Japan show an 18 and 12% prevalence respectively (R. Castro, et al. J Med Genet. 2004;41:454-458).
The A1298C mutation, on the other hand, does not show as much population variance; its prevalence is more uniform within the currently studied groups. The table below notes the variant frequencies lumping the heterozygous and homozygous genotypes together.
For the most part, the other MTHFR gene mutations are still under study and their effects are not completely understood.
Geneticists and evolutionary biologists hypothesize that, in the past, mutations in MTHFR may have conferred benefits for people living in areas with higher incidence of malaria and tuberculosis. Some mutations may also play a role in protection from colon cancer and acute lymphocytic leukemia. It is interesting to note, however, that geneticists have not been able to identify any clear evolutionary advantage for C677T nor A1298C, the two most relevant mutations for our current discussion.
A more in-depth look at the regional and ethnic variations, with subgroupings by wild type, heterozygotes and homozygotes, can be found in the Journal of Medical Genetics in an article authored by Wilcken and colleagues.
MTHFR Mutations & Disease Risk
The level of MTHFR enzyme activity in a given individual depends on the genotype variant he or she carries. For example, homozygotes for C677T have approximately a 70% reduction of normal MTHFR enzyme activity and heterozygotes have approximately a 40% reduction of normal enzyme activity, according to ARIP Laboratories/National Reference Laboratory.
Impaired MTHFR function has multiple negative impacts on DNA synthesis and repair, embryonic development, neurotransmitter synthesis, and cardiovascular risk factors.
Cardiovascular Disease: The normal recycling of homocysteine to methionine relies on 5-MTHF. If there is a defect in the MTHFR enzyme, the result is lower levels of 5-MTHF and consequently, elevated levels of homocysteine. Hyperomocysteinemia is an independent risk factor for cardiovascular disease including atherosclerosis, heart attack, stroke and venous thrombosis from increased blood clots (Varga, E. et al. Circulation; 2005; 111: e289-e293).
Currently, only the C677T mutation is thought to be associated with elevated homocysteine levels and thus a majority of the research has been done on this variant.
Cancer Risk: Appropriate DNA methylation is important for proper DNA replication. A disruption in methylation may occur due to a reduction 5-MTHF, which leads to a buildup of homocysteine. This eventually results in a drop in production of S-adenosylhomocysteine (SAMe) – an inhibitor of several methyltransferases.
Individuals with MTHFR mutations have altered DNA methylation, which is associated with changes in gene expression and could potentially influence oncogenic processes. One example is the 2.8-fold increased risk for endometrial cancer in women with the C677T homozygous genotype (Crott, J. et al. Carcinogenesis. 2004; 22(7): 1019-1025).
Defects in Developing Embryos: The mechanism for this concern actually comes from multiple consequences of a mutated MTHFR enzyme. Developing embryos may be adversely affected by toxic levels of homocysteine that result from MTHFR mutations. Altered DNA methylation may also have direct negative effects on gene expression and DNA synthesis. The main concerns here are neural tube defects and other midline defects such as cleft palate, although they are not the only ones. Noteworthy research includes a study from Ireland showing that 26% of all neural tube defects were related to either the homozygous or heterozygous MTHFR mutations (Kirke, P. et al. BMJ. 2004;328:1535-1536)
Additionally, a 2.6-fold increase in the frequency of the MTHFR C677T polymorphism has been observed in the mothers of Down Syndrome patients in South India (Cyril, C. et al. Indian J Hum Genet. 2009; 15(2): 60-64).
A very recent meta-analysis supports the association between recurrent pregnancy loss (RPL) and the C677T genotype in Asians, although this association was not found in Caucasians (Wu, X. Genet Test Mol Biomarkers. 2012 Feb 7).
Congenital heart disease (CHD) in children has also been linked to MTHFR gene mutations in either the mother or the child, although a complete analysis and conclusion is still unclear. One of the more recent studies however, did find a clear relationship between CHD and MTHFR mutations (Garcia-Fragoso, L. et al. Int J Genet Mol Biol. 2010; 2(3): 43–47).
The authors note that, “The prevalence of the TT polymorphism was higher in mothers (22%) than in controls (10%). Compound heterozygosity for both polymorphisms was 3.7 times more common in children with CHD than in the newborn controls. Mothers of children with CHD were more likely to be compound heterozygotes”
An interesting question in relation to embryonic development is whether it is the MTHFR mutation in the mother or in the developing child that is the critical determinant in the development of congenital defects. It is certainly possible that both mutations play a role. Further research is certainly needed to shed light on this very important area of prenatal and pregnancy health.
Neuropsychiatric & Neurological Conditions: MTHFR mutations have been linked to neuropsychiatric conditions due to the indirect effects of MTHFR activity on the production of serotonin, dopamine and norepinephrine, as well as the potentially toxic effect of hyperhomocysteinemia. Schizophrenia-like syndromes, bipolar disorder, Parkinson’s disease, Alzheimer’s disease and vascular dementia have all been associated with one or more mutations of the MTHFR gene (Lewis, X. Molecular Psychiatry. 2006;11, 352–360).
Insomnia, irritability, forgetfulness, endogenous depression, organic psychosis, peripheral neuropathy, myelopathy and restless leg syndrome are all also mentioned in the literature as potentially being influenced by this enzyme deficiency. The MTHFR C677T homozygous genotype has also been associated with an increased risk for migraine with aura in most ethnic groups except for Caucasian populations (Schurks M., et al. Headache. 2010; 50(4):588-99).
In a recent metanalysis, there was a relationship between the C677T mutation and increased susceptibility for depression (Lewis, X. Molecular Psychiatry. 2006;11, 352–360).
Understanding the indirect connection between MTHFR mutation and neurotransmitter production opens up the possibility of using folate supplementation alone or as an adjunct to medications as a treatment for depression.
Some studies indicate a connection between the C677T MTHFR mutation and increased risk of autism and ADHD. Case-control comparisons revealed significantly higher frequency of homozygosity as well as heterozygosity for both the C677T and A1298T genotypes among autistic versus non-autistic children (Liu X. et al. J. Autism Dev Disord. 2011;41(7):938-44). Interestingly, it is the A1298C genotype which may be associated with increased rates of ADD/ADHD, and not the C677T genotype (Gokcen, C. et al. Int J Med Sci. 2011; 8(7): 523–528).
Other Chronic Health Concerns: Other areas of recent study include the association between MTHFR mutation and epilepsy, Turner’s syndrome, infertility and inflammatory bowel disease. With regard to the latter, researchers have reported a 2.7 and 2.8-fold higher risk for Crohn’s disease and ulcerative colitis, respectively, in people who are homozygous for the C677T variant (Crott, J. et al. Carcinogenesis. 2004; 22(7): 1019-1025).
Therapeutic Implications
No number of medications or surgeries can “fix” a deficient enzyme. This is not to say the situation is hopeless, and patients must simply live with the risks and predispositions inherent in their genes.
Corrective treatment must move in the direction of optimizing each individual’s genotype expression. In the context of MTHFR mutations, the goal is to optimize as far as possible the production and function of this enzyme, to counterbalance the individual’s natural tendency for under-expression. This can be done through a nutrigenomics approach.
Some clinicians treat patients with MTHFR mutations by supplementation with 5-MTHF, the end product of MTHFR’s main catalytic reaction. Other vitamins and minerals that affect methylation cycles can also be helpful.
Before effective protocols can be designed, however, one must know what one is treating. MTHFR genetic tests are now readily available, but to date, there are no firm guidelines on who should be tested for MTHFR mutations.
My view is that it is wise to be vigilant when working with patients who fall into any of the above-mentioned chronic disease categories, or who have family members with known MTHFR mutations, or histories suggestive of this possibility. I also consider it when working with patients interested in preventative health programs and/or family planning.
You can learn a lot from a simple blood screening for MTHFR mutation at the C677T and A1298C points. Any patient who is positive for any form of MTHF genotype mutation should be counseled on the importance of repleting 5-MTHF. It’s usually a good idea to give information about MTHFR testing to other family members who may also carry mutations.
There is no advantage to waiting until a patient shows signs or symptoms of one of the MTHFR mutation-associated chronic diseases states; if there is a suspected family history or if a patient is not responding as would be expected to your usual therapeutic approaches – test and treat appropriately. What you discover could have big impact on someone’s future health.
END
Dr. Bianca Garilli is a former US Marine turned Naturopathic Doctor. She is the Director of Lifestyle Medicine at the Institute for Restorative Health in Davis, CA. In addition to her clinical practice, she also consults for nutritional supplement companies, teaches holistic nutrition at Hawthorn University, is active in writing for the professional and public audiences and routinely lectures on a wide variety of medical and wellness topics. Dr. Garilli specializes in natural and nutritional approaches to chronic disease with a focus on CFS, fibromyalgia, GI conditions, migraines, and ADD/ADHD. For further information, visit www.drbiancagarilli.com