 From the outside looking in, the dietary supplement world can seem like a bewildering place—an extensive selection of products, all using carefully worded terms to suggest that they can help with particular ailments; vast lists of unpronounceable herbs or ingredients; labels loaded with “nutrition facts” that most people don’t really understand.
From the outside looking in, the dietary supplement world can seem like a bewildering place—an extensive selection of products, all using carefully worded terms to suggest that they can help with particular ailments; vast lists of unpronounceable herbs or ingredients; labels loaded with “nutrition facts” that most people don’t really understand.
I’m sure many people wonder, “How is all of this being blended and manufactured? How is the quality and potency being verified?”
As an analytical chemist working for one of the nation’s leading practitioner focused supplement companies, I can comfortably say that a lot of work goes into the manufacturing and testing of a supplement before it goes to market.
It’s important to know that, because there’s still a widely held notion that the dietary supplement industry is unregulated. While it is true that poor quality raw materials and sub-standard products sometimes make it into the market, it is also true that the major brands are extremely diligent and scrupulous about their supply chain control, their quality control systems, and their analytical testing.
For me personally, this is not just a matter of professional pride. First and foremost, I am a health nut. I was a swimmer from 3rd grade through college, and I am currently part owner of a Crossfit gym. As a competitive powerlifter, I placed 3rd at the IPL (International Powerlifting League) World Championship in 2017 in the 67.5 kg weight class. It probably comes as no surprise that diet and nutritional supplements are a necessary part of my routine. I also have a Masters in Chemistry.
When I began working at Designs for Health in 2010, I found a place where I could combine my love for science and fitness in a truly helpful way. Since I was now directly involved in validation testing and quality assurance, it also removed any doubts I had about those overwhelming ingredient lists on the products I purchased for my own use.
From inside the analytical testing lab, I’d like to share some important aspects that I think all physicians should know about.
R&D: Prototyping and Blending a New Formula
A lot of time and effort goes into the development of a new supplement formula. First, the Product Development team establishes effective ingredients and dosages likely to give a desired benefit. The team then creates a Master Formula sheet, where the amounts of active ingredients are calculated to deliver the desired efficacy for the proposed label claim. The formulators must account for, among other factors, the potency, moisture, and physical properties of the ingredients.
Properties of Raw Materials: In the case of Vitamin B1 for example, our raw materials supplier may indicate that the material delivers 98% Thiamine HCl. However, this number represents the salt form and not the active form of Thiamine/Vitamin B1 for which our labels must declare a percent Daily Value (DV). So, our input of this ingredient needs to be adjusted based on molecular weight and the amount of moisture found in the raw material.
Then, there’s the fact that some materials can degrade over time from heat, humidity, light, and oxidizing agents. We have to calculate for that, and add overages of certain ingredients to maintain the desired shelf life. We’ll discuss this more fully, when we take a closer look at some of the struggles and challenges we faced in formulating our Primal Multi formula, which contains 43 different ingredients.
“Runability” is a term we use to mean the ease with which a particular formula can actually be produced on our manufacturing equipment. Simple formulas with only a few ingredients are easy to run. But for a formula like Primal Multi, which contains so many ingredients with varying physical characteristics (ex: density, flow index, particle size, and even level of static charge), runability is one of the most difficult issues we must face.
Selection of Excipients: The first step is to create a small-scale blend (1-500 grams) of the 40+ active ingredients. We then use this to select the excipient profile. As is the case with pharmaceuticals, dietary supplements also contain non-bioactive ingredients that enable the diverse active ingredients to be blended, bound together, and stabilized.
Excipients such as silicon dioxide and tricalcium phosphate function to adjust flow rates of the ingredients through the machinery. Others, such as stearic acid or magnesium stearate, add lubrication. Still others, like microcrystalline cellulose and dicalcium phosphate, allow powders to bind. All of these aid in the manufacturing process, but they need to be chosen carefully.
Through several trial runs using test formulations, our team determined that the excipient profile for Primal Multi would include 8.71% microcrystalline cellulose as the binder, 0.33% silicon dioxide for flow adjustment, and 0.57% magnesium stearate for lubrication. They tried many other compositions, but these yielded inferior performance, inadequate binding, and poor flow characteristics.
Determining Blend Times: Once the team found the optimal composition of active and excipient ingredients, the next step was to create a scaled-up version of the formula (8-10 kilograms). This is more than ten times the size of the original bench blend. This is mixed in a double cone blender to investigate homogeneity of the blend. During the blending process, we take samples at multiple time points from various locations in the mixer, and we test for the presence of specific marker compounds.
For the Primal Multi product, we selected 8 ingredients for testing: Vitamin K1, Vitamin K2 (MK-4), Selenium, Vanadium, Calcium, Quercetin, Resveratrol, and Hesperidin. They were chosen because they represent a large range of concentrations (microgram to milligram amounts). The time point that most closely matched the desired label claim for all of these ingredients was 5.5 minutes from the start of mixing. This was designated as the best blend time for Primal Multi.
With the blend time determined, we then created another scaled up version of the formula and then we tested everything to verify the label claim…yes, I mean everything. All 43 ingredients!
Scaling Up for Production: When scaling up from a cone blender to the actual production blender, we need to consider a few additional parameters. The difference in size from the cone blender to production is about 44 times, so that means 10 kilograms becomes 440 kilograms. The blend time and batch size are mathematically calculated based on equipment rotational speed and blend powder density.
In the case of Primal Multi, the 5.5-minute blend time determined in early blending steps was adjusted to 16.5 minutes of mixing time in actual production. Then, we have to run samples on an encapsulation machine to determine the optimal settings (machine speed, dosing disc size, and tamping pin settings) to achieve the targeted capsule fill weights.
Packaging Considerations: We select our packaging components based on the serving sizes and capsule doses required to deliver the intended benefit. Depending on the physical properties of the individual ingredients in a given formula, we decide whether a dessicant is necessary to protect the product against moisture.
Once a formula is produced and bottled in its finished form, we test it yet again to validate the presence of the active ingredients. Figure 1: HPLC in stacked configuration: Degasser, Pump, Sampler, Column Compartment, Detector
This leads to the next question: How do we actually verify these different analytes in the product?
Chromatography & Analytic Methods
As mentioned previously, a lot of dietary supplement—especially multivitamin formulas—boast large lists of ingredients. Chemical analysis of all these diverse ingredients can be very challenging.
One of the most common forms of analytical testing is called High Performance Liquid Chromatography (HPLC).
This instrument is used to separate components of a mixture, and then identify and quantify them compared to a purified reference standard of the analyte(s) of interest.
The main components of the HPLC unit are the degasser, pump, sampler, column compartment, and a detector (Figure 1). As you would expect from the name, everything is prepared in a liquid medium; this means that full dissolution of the compounds being analyzed is very important.
The equipment takes a liquid solvent containing the sample mixture and pumps it through a column that contains solid adsorbent material. Because ingredients all have unique chemical structures, they will interact differently with this adsorbent material. Some will be retained longer, so the components will all reach the detector at different points in time.
Once through the detector, the analytes will be seen as “peaks” (Figure 2) and we can determine the concentration of each analyte based on the peak area in the sample compared to the peak area of the purified standard run.
This process may sound simple, but the unfortunate thing is that there is no single “magic method” that can test for all possible ingredients.
Much of the work happening in the world of analytical chemistry and HPLC is focused on development of methods, particularly as new ingredients and raw materials emerge in the market all the time.
Figure 2: HPLC Chromatogram depicting various B vitamins in a multivitamin formula.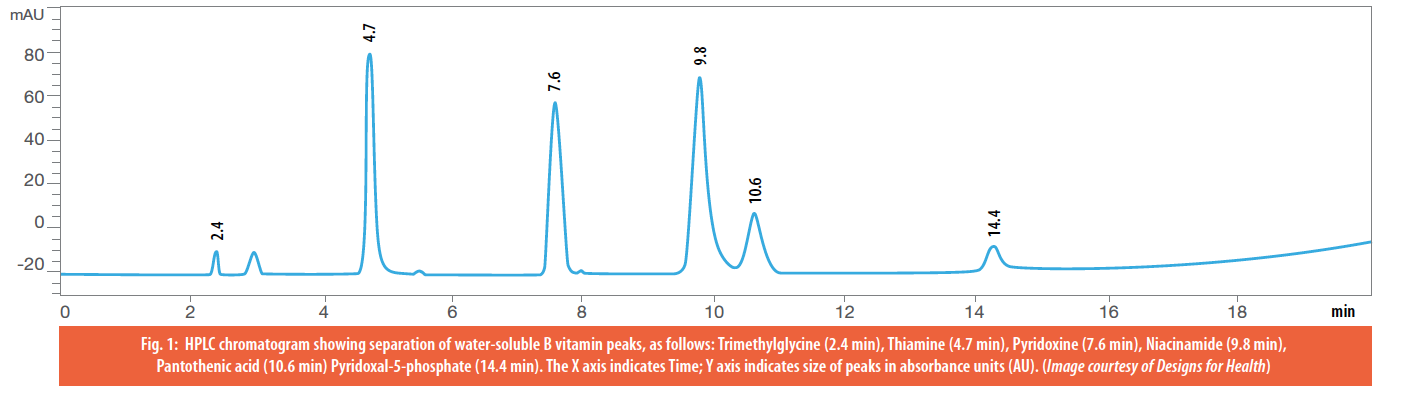
Controlled storage chamber used for accelerated stability testing of supplement products at Designs for Health. The chamber allows quality assurance technicians to test ingredient integrity after exposure to high temperatures and humidity levels.
Stability: Not All Ingredients are Created Equal
We use HPLC testing to verify raw material potency and to validate consistency of blends, but it can also detect changes in ingredients over time. This is important, because most raw materials are subject to some degree of potential degradation over time. Some are very fragile and highly susceptible to degradation; others are much more stable.
Our raw materials vendors will typically give us a Certificate of Analysis on the stability of their materials, but these figures represent the materials in isolation, under ideal storage conditions. How these ingredients hold up in a finished product blend or under different storage conditions is a different story.
Stability testing to generate an expiration date is common in the supplement industry. This involves simulating a long turnover time of 2 years under various sub-optimal conditions. We do this by subjecting our formulas to accelerated stability testing, where they are exposed to high temperatures and humidity (40o C and 75% Relative Humidity) in a controlled storage chamber (Figure 3). One week under these conditions is equivalent to one month of real time in ambient conditions.
Over the years of testing, analytical chemists in our industry have discovered many of the characteristics and tendencies of particular ingredients commonly used in supplement formulas.
For example, we know that Vitamin B1 is highly susceptible to heat and moisture. Figures 4A and 4B are HPLC chromatograms of the same multivitamin formula. But the test shown in Fig. 4B was run after the formula had been in an accelerated stability chamber for more than 20 weeks.
You can clearly see a decrease and change in shape of the Vitamin B1 peak at a retention time of 4.7 minutes, along with the development of other peaks, which likely represent degradation products of Vitamin B1. Note that there is an order of magnitude difference in the scale of the Absorption Units (Y axis) between the two chromatograms. Figure 4A: HPLC Chromatogram of Thiamine (Vit B1) before exposure to heat and humidity.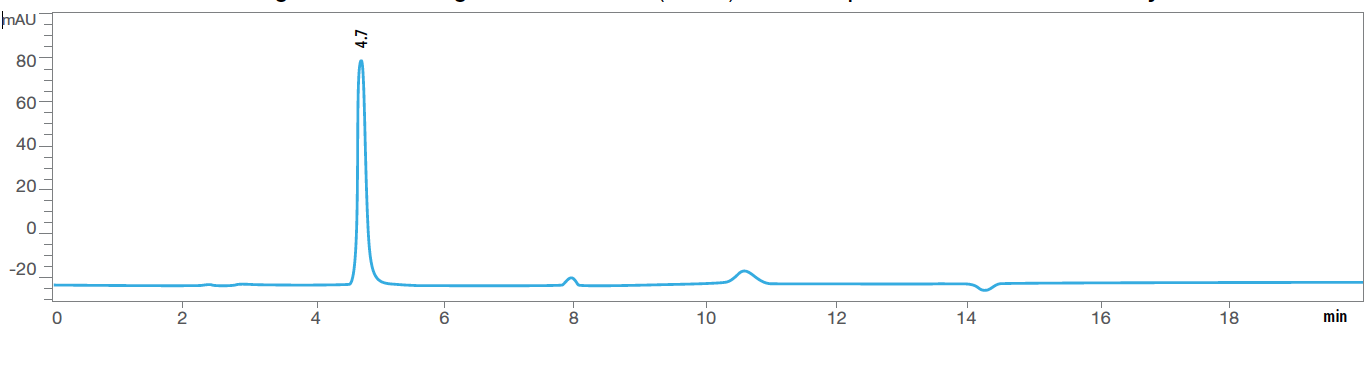
Figure 4B: HPLC Chromatogram of Thiamine (Vit B1) after exposure to heat and humidity.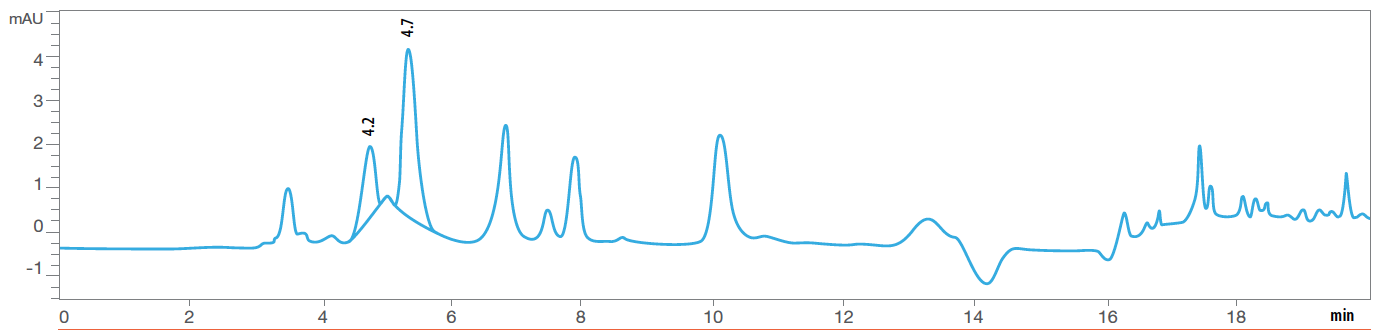
In addition to the products themselves, we also use the accelerated chamber to evaluate different types of bottles. There are clearly some bottling materials that are superior to others in their ability to protect vulnerable ingredients.
For example, Vitamin D3 is another ingredient that degrades on exposure to high temperature and humidity. Recent studies have shown that Vitamin D3 retains its integrity much better in glass bottles compared with high density polyethylene (HDPE) bottles.
All of this testing allows for better planning when prototyping blends.
In the case of our Primal Multi, we adjusted the formula to add an overage to the quantities of vitamin B1 and D3. This offsets any potential degradation that might happen during shipping or routine customer use. We also selected amber glass bottles, to provide optimal protection for the most vulnerable ingredients.
Once we finalize the formulation and packaging, we continue to conduct stability tests on our completed products in their finished form, to ensure that they meet label claim over time.
From the idea for a new formula in the minds of our product development team, to the consideration of the many intricate manufacturing parameters, to the verification of ingredients and their stability in the analytical lab, Designs for Health consistently lives up to its motto of “Science First”.
END







